if there is any reaction, both are transformed" - Carl Gustav Jung
Catalysis
Under the umbrella of the Northwestern Institute for Sustainability and Energy, a variety of projects are aimed at enabling energy efficient approaches to large scale chemical processes.
The group of scientists with whom we collaborate includes members of the Chemistry, Chemical Engineering, and Materials Science and Engineering Departments. We also work closely with scientists at Argonne National Laboratory, which is a world-class facility with which we have unique proximity.
Our work in the space of catalysis has primarily been aimed at heterogeneous catalysis, which is applicable for applications in transportation, power generation, and industrial chemical processes. Additionally, there is increasing concern with the environmental impact of these activities. An area of emphasis is to enable the successful transition to renewable energy sources through the development of new materials and the reduction of deleterious byproducts such as pollutants and greenhouse gases. Improvements in processing carbon-neutral chemical sources of energy, particularly biomass, will depend strongly upon advances in this field.
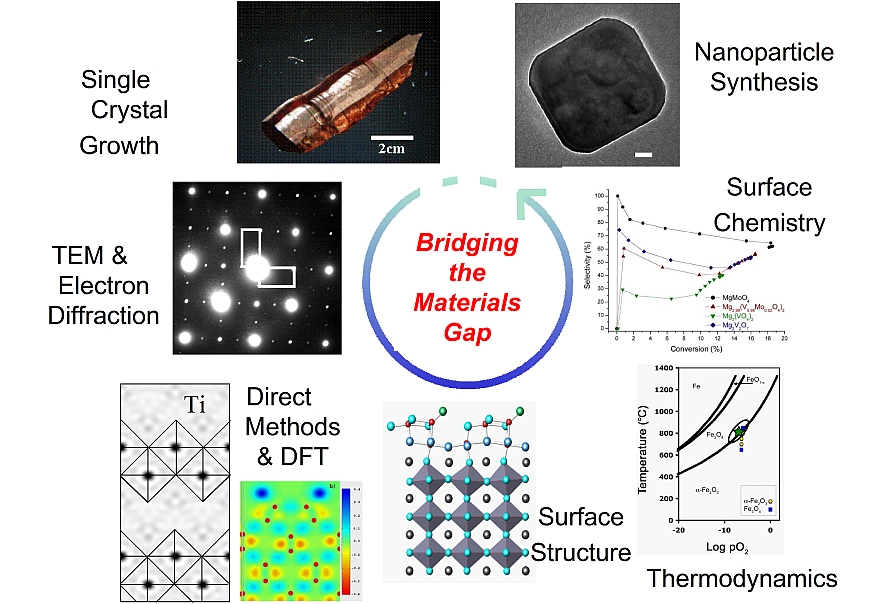
Towards this end, we use a fundamental science approach. We use a wide range of techniques ranging from theoretical modeling of materials properties and wet chemical synthesis of nanoparticles to reactor studies to probe catalytic activity and selectivity. A full toolbox of characterization tools is employed, including XPS, XRD, FTIR, Raman, and TEM. We aim to bridge the "Materials Gap", to connect work done on single crystal surfaces under controlled conditions (generally homogeneous systems) with heterogeneous catalysts consisting of metallic nanoparticles on well-faceted nanoparticle oxide supports.
In this effort, we are investigating the effect that the nanoparticle support can have on the overall catalytic performance of the system. For example, the lattice matching between a metal nanoparticle and its oxide support affects the Winterbottom shape of the nanoparticle. In turn, the shape of the nanoparticle can affect the catalytic performance; acrolein hydrogenation has a higher selectivity towards alcohols on the facets of Pt than the edges or corners, due to the higher coordination number of the facets. Other variables being explored are the acidity, ionicity, and band structure of the oxide support.
Related Presentations:
Recent Publications:
-
Segregation in Bimetallic Nanoparticles
L. Peng, Emilie Ringe, R. P. Van Duyne and L. D. Marks
Phys. Chem. Chem. Phys. DOI: 10.1039/c5sp01492a
-
Imaging the Atomic Surface Structures of CeO2 Nanoparticles
Lin, Y., Z. Wu, J. Wen, S.H. Overbury, K.R. Poeppelmeier, and L.D. Marks
Nano Letters, 2014, 14(1), 191-196
-
In situ XANES study of methanol decomposition and partial oxidation to syn-gas over supported Pt catalyst on SrTiO3 nanocubes
Wang, H., J.L. Lu, C.L. Marshall, J.W. Elam, J.T. Miller, H.B. Liu, J.A. Enterkin, R.M. Kennedy, P.C. Stair, K.R. Poeppelmeier, and L.D. Marks
Catalysis Today, 2014. 237, 71-79
-
A chemical approach to understanding oxide surfaces
J. A. Enterkin, A. E. Becerra-Toledo, K. R. Poeppelmeier, and L. D. Marks
Surface Science 606 (2012) 344
-
Oriented Catalytic Platinum Nanoparticles on High Surface Area Strontium Titanate Nanocubes
J. Enterkin, K. R. Poeppelmeier and L. D. Marks
Nano Letters 11 (2011) 993
-
Propane oxidation over Pt/SrTiO3 nanocuboids
J. A. Enterkin, W. Setthapun, J. W. Elam, S. T. Christensen, F. A. Rabufetti, L. D. Marks, P. C. Stair, K. R. Poeppelmeier and C. L. Marshall
ACS Catalysis 1 (2011) 629
-
Wulff Construction for Alloy Nanoparticles
E. Ringe, R. P. Van Duyne, and L.D. Marks
Nano Letters 11 (2011) 3399
-
A homologous series of structures on the surface of SrTiO3 (110)
J.A. Enterkin, A.K. Subramanian, B.C. Russell, M.R. Castell, K.R. Poeppelmeier and L.D. Marks
Nature Materials 9 (2010) 245.
